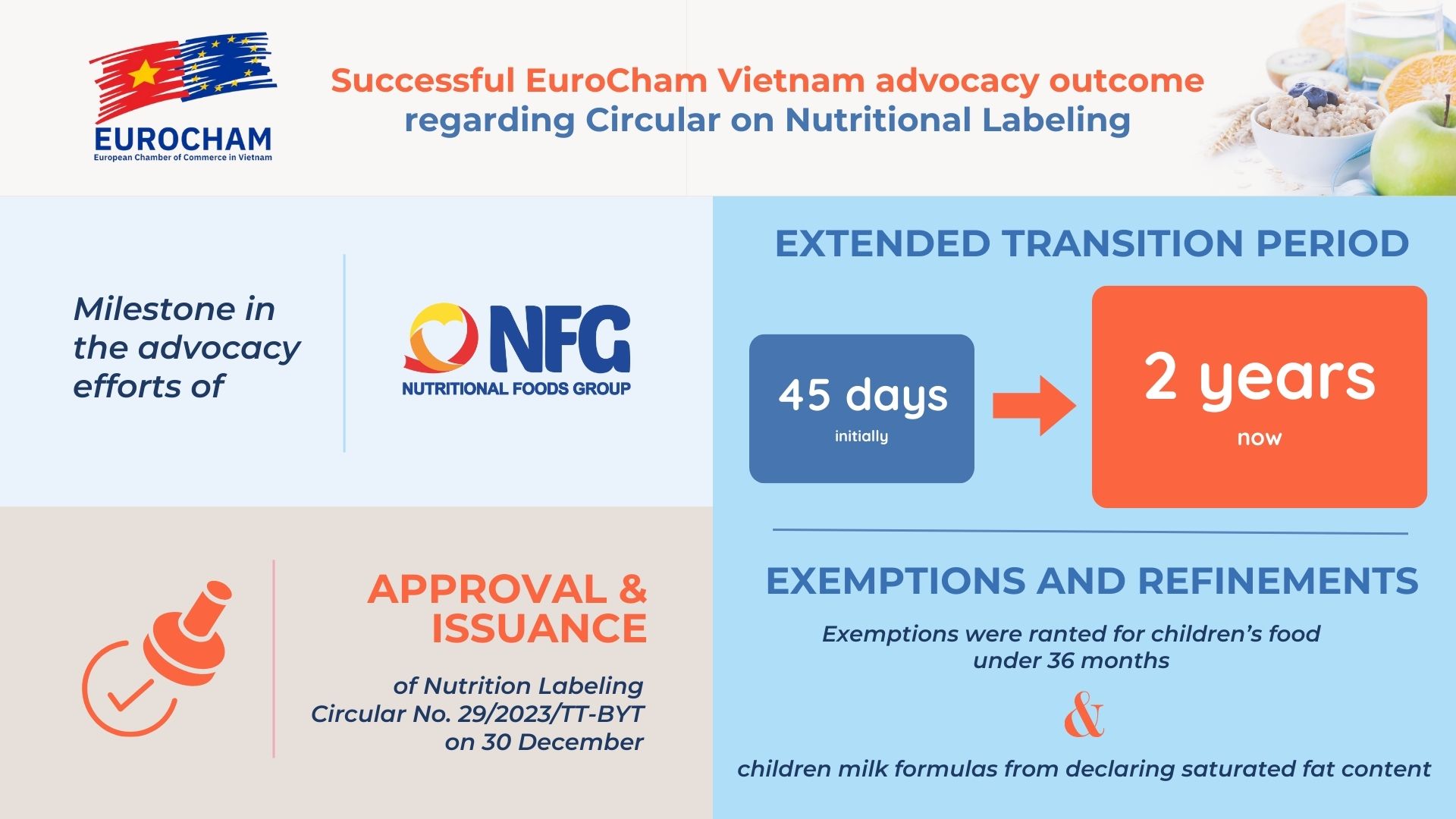
As the calendar turns to 2024, EuroCham Vietnam is delighted to share a significant milestone in the advocacy efforts of our Nutritional Foods Group Sector Committee — the approval and issuance of Nutrition Labeling Circular No. 29/2023/TT-BYT on 30 December. This achievement represents the culmination of a two-year engagement focused on the Ministry of Health’s (MOH) draft Circular on Nutrition Labeling, which initially mandated all food products to adopt new labels within a stringent 45-day timeframe from the signing date for production or importation.
Initially, the MOH required compliance within a narrow 45-day window. This approach:
- Created complexities in the seven-stage process that encompassed nutritional testing, label design, registration, production, and import customs clearance;
- Led to non-compliance and legal complications due to the inherent variability in nutritional compositions of agricultural products; and
- Disrupted supply chains, with a significant impact on nutritional products for infants, which typically require meticulous planning months in advance.
On 20 October 2023, EuroCham, in collaboration with AmCham and the Vietnam Dairy Association, raised concerns about the 45-day compliance window in letters to the Prime Minister, Deputy Prime Minister, and the Minister of Health. These collective efforts yielded results on 24 November 2023, as the MOH held consultations with the same stakeholders to finalize the circular. During this meeting, an open dialogue addressed the challenges posed by rushed labeling changes.
Through persistent and solution-oriented discussions, EuroCham played a vital role in securing practical circular guidelines that greatly benefit our members. Key accomplishments include:
- Extended Transition Period: The Deputy Minister of Health embraced a 2-year transition period for new labeling, discarding the initial 45-day timeframe.
- Exemptions and Refinements: Exemptions were granted for children’s food under 36 months and children milk formulas from declaring saturated fat content. The requirement for declaring saturated fats was confined only to products containing added sugars.
These changes ensure a realistic implementation timeframe until 31 December 2025, and offer more feasible guidelines for specific food categories.
We extend our heartfelt thanks to the Ministry of Health for their cooperative spirit and willingness to engage in constructive dialogue. This was crucial in crafting a balanced and practical solution, highlighting the importance of ongoing collaboration in shaping Vietnam’s regulatory framework.