About our European Standards Medicines Sector Committee
The European Standards Medicines Sector Committee, formerly known as International Quality Medicines (IQMED) – Generic & Biosimilar, was established in October 2016 under EuroCham’s Healthcare Forum. This Sector Committee has created a forum where business professionals from interest-shared industries can discuss issues and solutions to provide affordable, high-quality, and sustainable supply sources for branded generic drugs.
The activities we carry out are driven by a shared mission, focused on:
- Delivering affordable, high-quality, sustainable branded generic medicine and services to Vietnamese people;
- Collaboration with key stakeholders such as the government, providers, and payers in Vietnam to build efficient legal frameworks and implementation platforms.
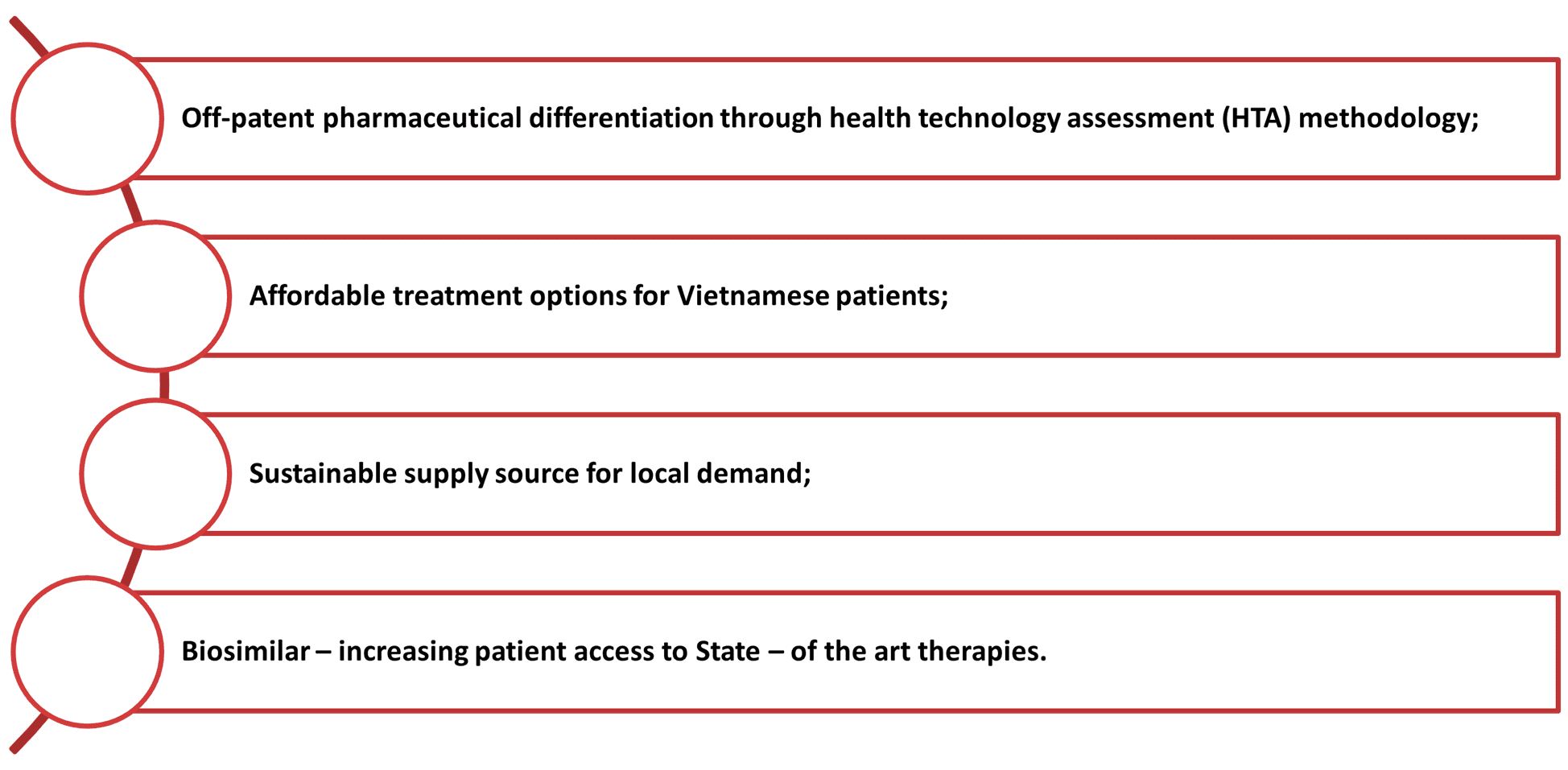
Key Interests
Leadership

Gregory Charitonos
CHAIRMAN
Medochemie (Far East)

Attila Molnar
VICE CHAIRMAN cum. Treasurer
Egis PHARMACEUTICALS

Aleksandrs Parfjonovs
VICE CHAIRMAN
Grindeks

Magdalena Krakowiak
VICE CHAIRwoMAN
Adamed PHARMA VIETNAM
European Standards Medicines Sector Committee Members
News
May 2024: Sent Joint Letter to MPI on investment acceleration
Board Meeting every quarter
Key Interest:
– Draft Pharma Law Amendment
– Circular 08/2022/TT-BYT
– Generics and Biosimilar Drug
Join the European Standards Medicines Sector Committee
European Standards Medicines membership requires the following criteria:
- Member of EuroCham.
- Must be foreign-invested or an international pharmaceutical company with a representative office in Vietnam. If the Member has a parent company, the parent company must also be a foreign company. [N.B. Separate legal entities belonging to the same multinational company shall be deemed to constitute a single company.]
- Must have product marketed in minimum three (3) markets in European Union (EU), Japan, Australia and New Zealand; and all together minimum ten (10) countries in Asia Pacific region, EU and North America.
- Must either:
- Own generic and biosimilar products manufactured at a manufacturing site in a SRA country
- Have at least one (1) manufacturing site in a SRA country
Membership applications must be completed, signed and sent to sec.comms@eurochamvn.org. The European Standards Medicines Bylaws must also be carefully reviewed.
Membership Fee: 3,500 USD/year
For inquiries, kindly contact:
Ms. Dao Manh Thuy DUYEN
Email: duyen.dao@eurochamvn.org
Mobile: (+84) 989 441 580